What is an NDC or National Drug Code and how is the NDC related to my Medicare Part D plan coverage?
A National Drug Code (NDC) is a number that uniquely identifies your prescription medication, the drug manufacturer, the medication strength, and the packaging of your medication.
An NDC can be up to 11-digits long and is printed on the label of your prescription bottle or packaging.
The FDA uses a 10-digit 3-segment NDC that can have a configuration of either 4-4-2, 5-3-2, or 5-4-1. However, Medicare (CMS) uses an 11-digit NDC and so do we. Please remember that the assignment of a National Drug Code (NDC) (or National Health Related Item Code (NHRIC)) does not in any way denote United States Food and Drug Administration (FDA) approval of the prescription drug.
An NDC can be up to 11-digits long and is printed on the label of your prescription bottle or packaging.
The FDA uses a 10-digit 3-segment NDC that can have a configuration of either 4-4-2, 5-3-2, or 5-4-1. However, Medicare (CMS) uses an 11-digit NDC and so do we. Please remember that the assignment of a National Drug Code (NDC) (or National Health Related Item Code (NHRIC)) does not in any way denote United States Food and Drug Administration (FDA) approval of the prescription drug.
You may notice that your drug's NDC is shown on your prescription bottle as an 11-digit code (60505257808) or could be formatted as 00000-0000-00 where the first set of numbers (up to 5) identifies the manufacturer (labeler), the second set of numbers (up to 4) identifies the product and strength, and the third set of numbers (up to 2) identifies the packaging. If any of the three sections have less than the maximum digits (5-4-2), you would put leading 0s on the front of the value.
So, 1234-123-1 would become 01234012301 when using the NDC on the Q1Medicare.com Drug Finder tool (Q1Rx.com).
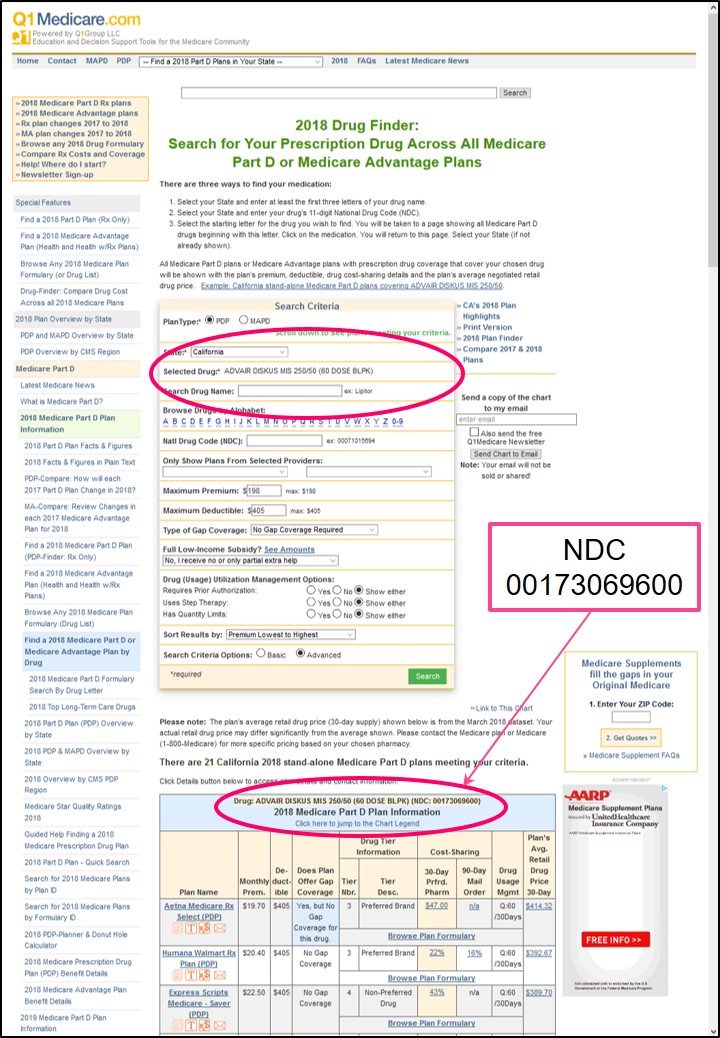
We use the 11-digit NDC in our Medicare Part D Formulary Browser and Q1Rx Drug Finder data so that people know that the information we show exactly represents the medications they are using. You can click here for an example search for NDC 00173069600 using our Q1Rx Drug Finder.
Below is a graphic showing the example results of a Q1Rx Drug Finder search for "ADVAIR DISKUS MIS 250/50 (60 DOSE BLPK)" represented by NDC 00173069600 across all Medicare Part D plans (PDP) in California.
You can see the NDC is listed along with the drug name, strength, and packaging information.
You can also see on our Q1Rx Drug Finder search form that you are able to enter either the drug name into the search field, "Search Drug Name" - or enter your NDC into the search field "Natl Drug Code (NDC)" and arrive at the same result.
For more information and regulatory guidance, please also see "What is the National Drug Code (NDC), how is it assigned, and what are its requirements?" (www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=207.33) where the federal regulations state in part:
You can also see on our Q1Rx Drug Finder search form that you are able to enter either the drug name into the search field, "Search Drug Name" - or enter your NDC into the search field "Natl Drug Code (NDC)" and arrive at the same result.

For more information and regulatory guidance, please also see "What is the National Drug Code (NDC), how is it assigned, and what are its requirements?" (www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=207.33) where the federal regulations state in part:
"(a) What is the NDC for a drug and what products must have unique NDCs?
The NDC for a drug is a numeric code. Each finished drug product or unfinished drug subject to the listing requirements of this part must have a unique NDC to identify its labeler, product, and package size and type.
(b) What is the format of an NDC?
(1) Except as described in paragraph (b)(4) of this section, the NDC must consist of 10 or 11 digits, divided into three segments as follows:
(i) The first segment of the NDC is the labeler code and consists of 4, 5, or 6 digits. The labeler code is assigned by FDA.
(ii) The second segment of the NDC is the product code and consists of 3 or 4 digits, as specified in paragraphs (b)(2) and (3) of this section.
(iii) The third segment of the NDC is the package code and consists of 1 or 2 digits as specified in paragraphs (b)(2) and (3) of this section. The package code identifies the package size and type of the drug and differentiates between different quantitative and qualitative attributes of the product packaging.
(2) The following combinations of labeler code, product code and package code character lengths are permissible:
(i) If a labeler code is either 5 or 6 digits in length, it may be combined with:
(A) A product code consisting of 4 digits and a package code consisting of 1 digit for a total NDC length of 10 or 11 digits (5-4-1 or 6-4-1), or
(B) A product code consisting of 3 digits and a package code consisting of 2 digits for a total NDC length of 10 or 11 digits (5-3-2 or 6-3-2).
(ii) If a labeler code is 4 digits in length, it may be combined only with a product code consisting of 4 digits and a package code consisting of 2 digits for a total NDC length of 10 digits (4-4-2).
(3) A registrant or private label distributor with a given labeler code must use only one Product-Package Code configuration (e.g., a 3-digit product code combined with a 2-digit package code or a 4-digit product code combined with a 1-digit package code). This single configuration must be used in all NDCs that include the given labeler code that are reserved in accordance with 207.33(d)(3) or listed in accordance with 207.49 or 207.53. . . ."
Browse FAQ Categories
Ask a Pharmacist*
Have questions about your medication?
» Answers to Your Medication Questions, Free!
Available Monday - Friday
8am to 5pm MST
8am to 5pm MST
*A free service included with your no cost drug discount card.
Q1 Quick Links
- Sign-up for our Medicare Part D Newsletter.
- PDP-Facts: 2024 Medicare Part D plan Facts & Figures
- 2024 PDP-Finder: Medicare Part D (Drug Only) Plan Finder
- PDP-Compare: 2023/2024 Medicare Part D plan changes
- 2024 MA-Finder: Medicare Advantage Plan Finder
- MA plan changes 2023 to 2024
- Drug Finder: 2024 Medicare Part D drug search
- Formulary Browser: View any 2024 Medicare plan's drug list
- 2024 Browse Drugs By Letter
- Guide to 2023/2024 Mailings from CMS, Social Security and Plans
- Out-of-Pocket Cost Calculator
- Q1Medicare FAQs: Most Read and Newest Questions & Answers
- Q1Medicare News: Latest Articles
- 2025 Medicare Part D Reminder Service
